Analyzing Stainless Steel Flanges in LNG Gasification Stations (Part one)
1.1 Analysis of failure mechanism of corrosion
Austenitic stainless steel 304 has excellent corrosion resistance and weldability. Studies have shown that the main forms of corrosion of austenitic stainless steel 304 welding seam are intergranular corrosion and stress corrosion.
1. 1. 1 Intergranular corrosion
In a certain temperature range between 450 and 850°C, the supersaturated carbon in the austenite will combine with the chromium in the solid solution to form Cr23C6 carbides that precipitate at the grain boundaries (Figure 5). The diffusion rate is faster than that of chromium. Therefore, the chromium in the solid solution around the carbide is greatly reduced due to the precipitation of Ciz C6 carbide, that is, in the grain boundary and its adjacent area, and the chromium in the grain has no time to diffuse to the grain boundary, thus forming a chromium-depleted area. When the chromium content is lower than the chromium content required for passivation (mass fraction is about 12%), the passive state is destroyed, the potential drops, and the intragranular remains passive, thus forming a large cathode (matrix) and a small anode (the chromium-depleted area of the grain boundary area) of the microcouple battery, and accelerating the corrosion of the grain boundary.
The precipitation temperature of carbide precipitation (Cr23C6) is between 450 and 850°C, which is the sensitization temperature range of stainless steel intergranular corrosion, also known as the dangerous temperature range.
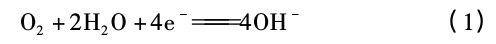
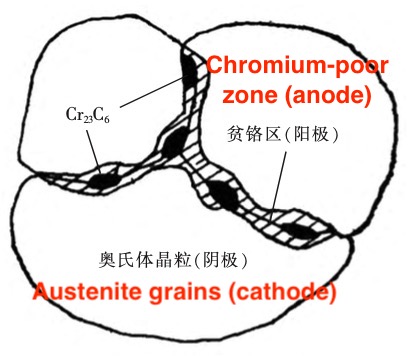
Figure 5 The precipitation of CR23C6 carbide
1.1.2 Stress corrosion
Under the joint action of stress and a specific corrosive medium, metal components are corroded and lead to brittle fracture, which is called cracking caused by stress corrosion. Stress corrosion is a special corrosion, and cracking is the final result of stress corrosion. There are two types of cracks. One is the crack developing along the grain boundary and the other is cracks developing in a different part. The first one is called intergranular cracking, with cracks passing through the grains, called transgranular cracking. The second one is the mixed type. For example, the main crack is the intergranular type, and the branch crack or tip is the transcrystalline type, which is one of the most dangerous forms of corrosion and can cause sudden accidents.
For austenitic stainless steel 304, the initial stress required for stress corrosion cracking of chloride ions is low, so it can be considered that sufficient stress always exists, whether it is applied stress, stress from thermal cycling or residual stress of welding. Unless annealing is performed, stress corrosion cracking of chloride ions easily occurs in welding zones where there is residual stress.
1.2 The analysis of the chemical composition
C is the main element causing intergranular corrosion. When the mass fraction of C is below 0.08%, the amount of C that can be precipitated is small; when the mass fraction of C is above 0.08%, the amount of C that can be precipitated increases rapidly. With the increase in C content in stainless steel, the chromium carbide generated at the grain boundary increases accordingly. As a result, the opportunity to form a chromium-poor region at the grain boundary increases, resulting in an increased tendency of intergranular corrosion, so carbon is the most harmful element against anti-intergranular corrosion. When the Cr content increases, it is beneficial to balance the chromium content in the chromium-deficient area and the chromium-rich area, thereby reducing the susceptibility to intergranular corrosion.
Table 1 Chemical composition of metals
Austenitic stainless steel 304 has excellent corrosion resistance and weldability. Studies have shown that the main forms of corrosion of austenitic stainless steel 304 welding seam are intergranular corrosion and stress corrosion.
1. 1. 1 Intergranular corrosion
In a certain temperature range between 450 and 850°C, the supersaturated carbon in the austenite will combine with the chromium in the solid solution to form Cr23C6 carbides that precipitate at the grain boundaries (Figure 5). The diffusion rate is faster than that of chromium. Therefore, the chromium in the solid solution around the carbide is greatly reduced due to the precipitation of Ciz C6 carbide, that is, in the grain boundary and its adjacent area, and the chromium in the grain has no time to diffuse to the grain boundary, thus forming a chromium-depleted area. When the chromium content is lower than the chromium content required for passivation (mass fraction is about 12%), the passive state is destroyed, the potential drops, and the intragranular remains passive, thus forming a large cathode (matrix) and a small anode (the chromium-depleted area of the grain boundary area) of the microcouple battery, and accelerating the corrosion of the grain boundary.
The precipitation temperature of carbide precipitation (Cr23C6) is between 450 and 850°C, which is the sensitization temperature range of stainless steel intergranular corrosion, also known as the dangerous temperature range.

Figure 5 The precipitation of CR23C6 carbide
1.1.2 Stress corrosion
Under the joint action of stress and a specific corrosive medium, metal components are corroded and lead to brittle fracture, which is called cracking caused by stress corrosion. Stress corrosion is a special corrosion, and cracking is the final result of stress corrosion. There are two types of cracks. One is the crack developing along the grain boundary and the other is cracks developing in a different part. The first one is called intergranular cracking, with cracks passing through the grains, called transgranular cracking. The second one is the mixed type. For example, the main crack is the intergranular type, and the branch crack or tip is the transcrystalline type, which is one of the most dangerous forms of corrosion and can cause sudden accidents.
For austenitic stainless steel 304, the initial stress required for stress corrosion cracking of chloride ions is low, so it can be considered that sufficient stress always exists, whether it is applied stress, stress from thermal cycling or residual stress of welding. Unless annealing is performed, stress corrosion cracking of chloride ions easily occurs in welding zones where there is residual stress.
1.2 The analysis of the chemical composition
C is the main element causing intergranular corrosion. When the mass fraction of C is below 0.08%, the amount of C that can be precipitated is small; when the mass fraction of C is above 0.08%, the amount of C that can be precipitated increases rapidly. With the increase in C content in stainless steel, the chromium carbide generated at the grain boundary increases accordingly. As a result, the opportunity to form a chromium-poor region at the grain boundary increases, resulting in an increased tendency of intergranular corrosion, so carbon is the most harmful element against anti-intergranular corrosion. When the Cr content increases, it is beneficial to balance the chromium content in the chromium-deficient area and the chromium-rich area, thereby reducing the susceptibility to intergranular corrosion.
Table 1 Chemical composition of metals
| wC /% | Standard austenitic stainless steel 304 | Base materials of flange sealing surfaces | Base materials of stainless steel tubes |
| wSi /% | less than and equal to 0.08 | 0.180 | 0.074 |
| wMn /% | less than and equal to 1.00 | 0.385 | 0.524 |
| wP /% | less than and equal to 2.00 | 2.399 | 1.083 |
| wS /% | less than and equal to 0.035 | 0.0287 | 0.0323 |
| wCr /% | less than and equal to 0.030 | 0.0047 | 0.0019 |
| wNi /% | 18.00 to 20.00 | 16.284 | 17.112 |
| wC /% | 8.00 to 11.00 | 6.347 | 8.198 |
The C content of stainless steel flanges with corrosion cracking in the heat-affected zone is seriously exceeded. Because the C content exceeds the standard, the more C diffuses in the welding process, the more carbides are formed, and the C consumption is greater. In addition, the test found that the content of G in the stainless steel flange was lower than the standard value. The lower the Cr content is, the greater the susceptibility to intergranular corrosion becomes, which intensifies the precipitation of carbide precipitation (C2C6). The reduction of Cr content leads to more serious chromium deficiency in the heat-affected zone, and accelerates the tendency of intergranular corrosion in the heat-affected zone of stainless steel.
The role of the element Mn in austenitic stainless steel 304 is mainly to reduce the critical quenching speed of steel, increase the stability of austenite during cooling, inhibit the decomposition of austenite, and maintain the austenite formed at high temperature to room temperature. However, Mn and § form MnS greatly reducing the ability of austenitic stainless steel 304 to resist pitting corrosion and crevice corrosion. In addition, with the increase in Mn content, the thermal conductivity of austenite decreases sharply, and the coefficient of linear expansion increases, so that great internal stress is formed during rapid heating or cooling, and the cracking tendency of the workpiece increases. The Mn content in the sealing surface material of the stainless steel lap joint flange with corrosion cracking in the heat-affected zone exceeds the standard, which can increase the possibility of corrosion cracking of the stainless steel.
The element Ni in austenitic stainless steel 304 mainly plays an anti-corrosion role, but in the medium containing chloride ions, chromium-nickel austenitic steel is also prone to damage and corrosion due to the oxide film. The Ni content in the material of the sealing surface of the stainless steel flange is lower than the standard value, which leads to the weakening of the corrosion resistance of the oxide film on the surface of the stainless steel.
Related News
- Structural Design and Strength Analysis of a Deepwater Riser Suspension Flange
- Analysis of Heat Dissipation Losses in High-Temperature Flanges and Pipelines Used in Oil Refineries
- Failure and Crack Analysis of an EO/EG Unit Tower Inlet Flange
- Pipe Flange Bolt Tightening in LNG Projects: Key Considerations
- Ultrasonic Testing of High-Neck Flange Welds
- Underwater Flange Connection Methods for Submarine Pipelines
- Key Technologies for Pressure Vessel Testing and Flange Connection Design
- Installation of Main Bolts for Lap Joint Flange in High-Temperature Gas-Cooled Reactors
- Structural Design and Finite Element Analysis of Anchor Flanges
- Key Welding Technology for High-Neck Flange and Steel Pipe Joints
